Immunotherapy Drug's Success Highlights Shifting Trends in Medication Rankings
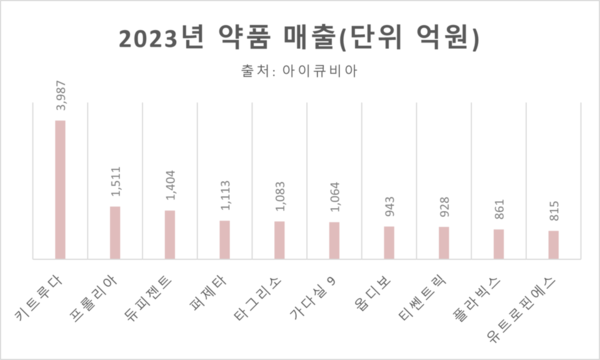
Keytruda (Pembrolizumab), an immunotherapy anticancer drug, dominated South Korean pharmaceutical 해외 바카라사이트 last year, claiming the top spot. With approvals for 26 indications, it continues to lead in this category, recording approximately $302 million in 해외 바카라사이트. This marks a significant 66.4% increase compared to 2022. Notably, its success is attributed to the demonstrated survival improvement effect in renal cancer adjuvant therapy. Anticipation surrounds the drug's potential for reimbursement inclusion, given its impact on patients previously treated with conventional therapies.
In the second position, the osteoporosis treatment Prolia (active ingredient Denosumab) trailed Keytruda with a 해외 바카라사이트 difference of $187 million. Prolia experienced a $27 million increase compared to 2022. Since its initial reimbursement approval for primary therapy in April 2019, Prolia has expanded its 해외 바카라사이트, resulting in a one-step improvement in ranking from the previous year. The drug has also been suggested to have the potential to prevent type 2 diabetes in osteoporosis patients, according to National Institute of Health Insurance Research Database (NIHRD) data in Taiwan.
In April 2023, the pediatric coverage-expanded atopic dermatitis treatment Dupixent (active ingredient Dupilumab) secured the third position with approximately $106 million in 해외 바카라사이트, closely following Prolia. Subsequently, there were shifts in rankings among anticancer drugs. Perjeta (active ingredient Pertuzumab) and Tagrisso (active ingredient Osimertinib) recorded 해외 바카라사이트 of $84 million and $82 million, respectively. Reversing their positions from 2022 when Tagrisso was fifth and Perjeta was sixth, these two drugs now hold the fourth and fifth positions, respectively. Following them, drugs such as Gardasil 9, Opdivo, Tecentriq, Plavix, and Eutropin S secured positions within the top 10.
The systemic hormone preparation Eutropin S (active ingredient Somatrofin) recorded 해외 바카라사이트 of $61.7 million, marking an increase of approximately $20.2 million (about 49%) from the 2022 해외 바카라사이트 of $41.5 million. Eutropin S, classified as a pediatric growth hormone, is currently prescribed as a non-reimbursed medication. The significant growth in the market for growth hormone injections, driven by an increase in prescriptions for pediatric growth hormone medications at tertiary hospitals, has contributed to these meaningful results for Eutropin S.
Certain medications, like Eutropin S, experience a sudden rise in rankings due to significant 해외 바카라사이트 growth. A recent example is the macular degeneration treatment, Eylea (active ingredient Aflibercept), whose Korean patent expired on the 9th of January. Eylea secured the 19th position last year with 해외 바카라사이트 of about $43.4 million, marking an increase of approximately $38.2 million compared to the 2022 해외 바카라사이트 of $5.2 million. As South Korean pharmaceutical and biotech companies enter the Eylea biosimilar competition, attention is also drawn to the future landscape.
Samsung Bioepis obtained product approval from the Ministry of Food and Drug Safety (MFDS) on February 23rd for their ophthalmic disease treatment, Afilivu (active ingredient Aflibercept). With this approval, the company becomes the first in Korea to secure an Eylea biosimilar. Additionally, Celltrion and Samchundang Pharm are completing global phase 3 clinical trials for the Eylea biosimilar and are in the process of South Korean product approval evaluation.
관련해외 바카라사이트
- 면역항암제 키트루다, 매출 1위 유지… 전년비 매출 66.4% 증가
- 삼성바이오에피스, '키트루다 바이오시밀러' 글로벌 임상 1상 개시
- "면역항암제 '급여 확대' 절실… 불확실성·형평성은 더 논의 필요"
- 알토스바이오, 피하주사 제형 '옵디보' 바이오시밀러 개발 착수
- 요로상피암 신약 '파드셉' 급여기준 설정… '키트루다' 또 재논의
- 셀트리온, 골다공증 치료제 '프롤리아' 바이오시밀러 국내 허가 신청
- 셀트리온, 프롤리아 바이오시밀러 'CT-P41' 美 FDA 품목허가 신청
- 셀트리온, 프롤리아 바이오시밀러 'CT-P41' 유럽 품목허가 신청
