February 29th: 25th Korea New 바카라사이트 쇼미더벳 Development Awards Ceremony
Yuhan Pharmaceuticals and GC Green Cross Clinch 'New 바카라사이트 쇼미더벳 Development Grand Prize' – Hanlim Pharmaceuticals Honored with Outstanding Award
Chong Kun Dang, GI-Innovation, and Onconic Therapeutics Receive 'Technology Export Awards' for Global Achievements

Yuhan Pharmaceuticals, the mastermind behind the non-small cell lung cancer treatment Leclaza, and GC Biopharma, the creative force behind the immunological disorder treatment 'Alyglo,' jointly secured the grand prize in the new 바카라사이트 쇼미더벳 development category at the awards ceremony. Hosted by the Korea New 바카라사이트 쇼미더벳 Development Association, led by Chairman Sung-Han Hong, the ceremony unfolded at Geranium Hall of the Seoul Samjung Hotel on February 29th, offering a platform to showcase accomplishments in new 바카라사이트 쇼미더벳 development and technology export.
During this prestigious event, Yuhan Pharmaceuticals was honored for the development of the non-small cell lung cancer treatment 'Leclaza,' while GC Biopharma received recognition for their immunological disorder treatment 'Alyglo,' both earning the grand prize in the new 바카라사이트 쇼미더벳 development category.
Additionally, Hanlim Pharmaceuticals, the brains behind the adjuvant for breast lesion removal surgery 'Luminomark Injection,' was presented with an outstanding award in the new 바카라사이트 쇼미더벳 development category. Furthermore, Chong Kun Dang, for their development of the histone deacetylase 6 inhibitor 'CKD-510 (development code),' GI-Innovation, for their development of the allergy treatment 'GI-301,' and Onconic Therapeutics, for their development of the gastroesophageal reflux disease treatment 'Zastaprazan,' were recognized with the Technology Export Award in the technology export category.
Chairman Hong expressed, "The New Drug Association will persist as a transformative control tower for new drug development, fostering communication and collaboration between the government and industry." He added, "Our goal is to expand the scope of the research and development (R&D) environment, ensuring that global achievements in new drug development receive the investment they deserve."
The Korea New 바카라사이트 쇼미더벳 Development Awards (KNDA), marking its 25th year, stands as South Korea's premier award in the field of new 바카라사이트 쇼미더벳 development, driven by the private sector with government support across various industries. Established by the KNDA in April 1999, its mission is to stimulate the growth of the South Korean biopharmaceutical industry and ignite enthusiasm for new 바카라사이트 쇼미더벳 R&D in the country.
Yuhan’s Leclaza and GC Biopharma’s ‘FDA New Drug’ Jointly Awarded; Hanlim’s Luminomark Inj. Receives Outstanding Recognition

The Leclaza tablet, a product of Yuhan Pharmaceuticals, is a groundbreaking novel 바카라사이트 쇼미더벳 designated as the 31st new 바카라사이트 쇼미더벳 in South Korea. Positioned as a third-generation EGFR tyrosine kinase inhibitor (TKI), Leclaza has gained approval for its use in the primary treatment of EGFR mutation-positive non-small cell lung cancer (NSCLC) and as a second-line treatment for T790M mutation-positive NSCLC following resistance to first and second-generation EGFR TKIs.

Se-Woong Oh, Head of the Central Research Institute at Yuhan Pharmaceuticals, shared, "We have collaborated with biotech to develop the early pipeline, and in the global late-stage clinical and marketing sectors, we are creating a business model through global partnerships." He explained, "In the case of Leclaza (active ingredient: Lazertinib), we acquired the candidate substance from Genoscience in 2015 and conducted the first clinical trial in 2017. In 2021, we received (conditional) approval from the Ministry of Food and Drug Safety (MFDS)."

Alyglo, developed by GC Biopharma, is an intravenous immunoglobulin preparation used for the treatment of 'primary immunodeficiency’. It is the eighth South Korean produced new drug to obtain approval from the U.S. Food and Drug Administration (FDA).
Alyglo is produced using a proprietary 'CEX Chromatography (Cation Exchange Chromatography)' purification process technology. This process effectively removes impurities such as blood coagulation factor XIa, a primary cause of thrombosis, maximizing quality and safety.
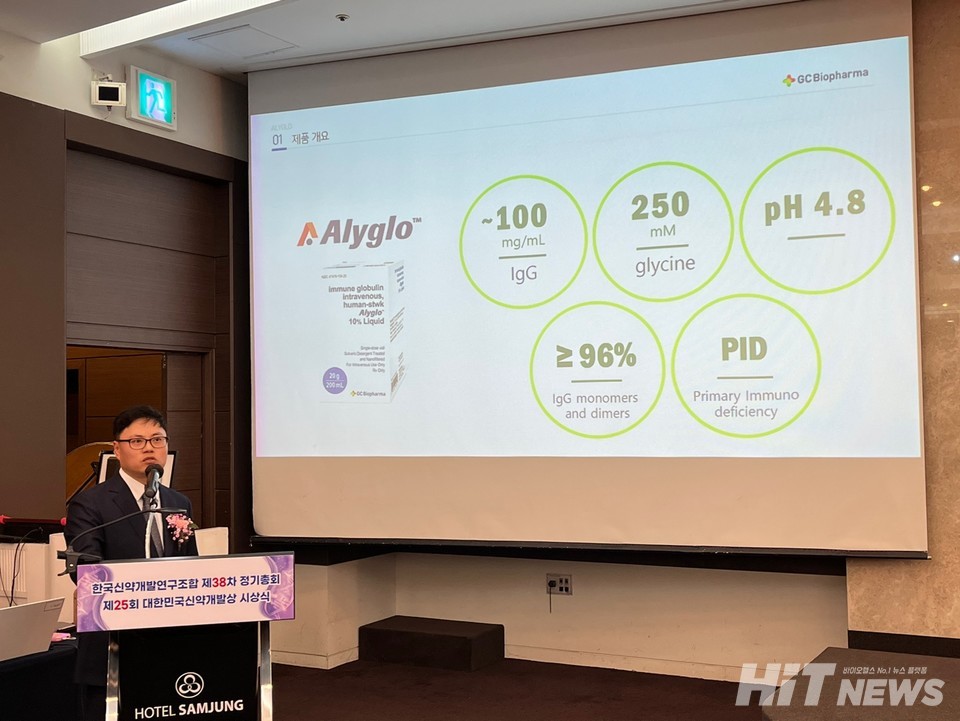
Kyung-Il Cha, Head of the Headquarters at GC Biopharma, stated, "GC Biopharma invests an annual R&D budget ranging from $98.7 million to $114 million. We embarked on the development of Alyglo to treat primary immunodeficiency," adding, "We are progressing with process development for Alyglo utilizing CEX Chromatography. The removal of thrombosis-inducing factors has enhanced its safety."
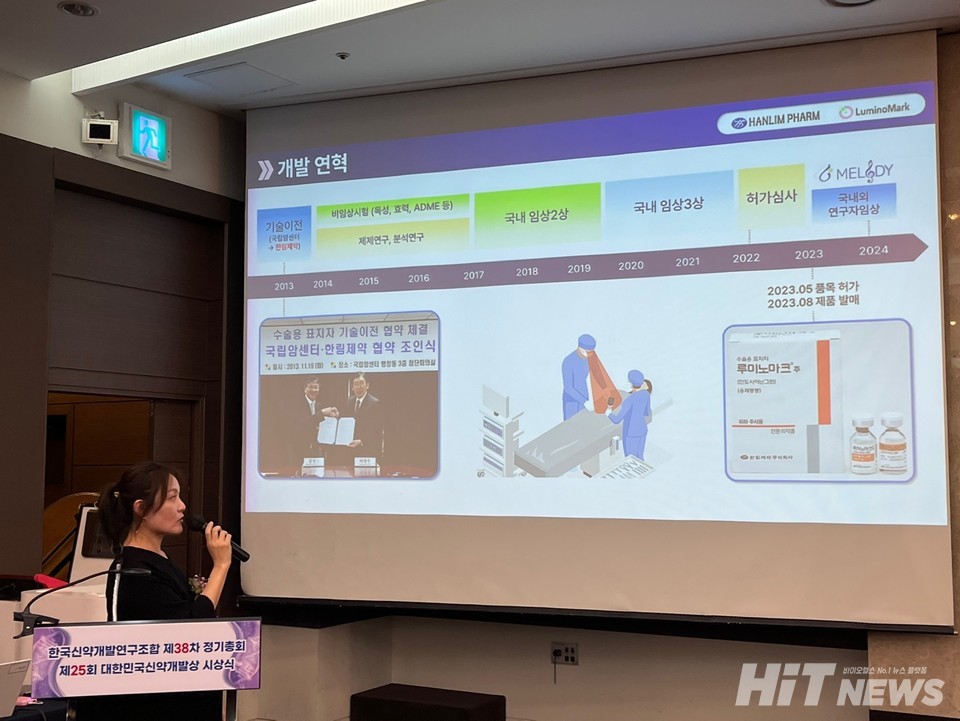
Luminomark injection, a creation of Hanlim Pharmaceuticals, is an innovative 바카라사이트 쇼미더벳 designed for surgical marking during breast lesion removal surgery, with 'Indocyanine Green' as its main component. Luminomark injection overcomes the drawbacks of pigment deposition and contamination, ensuring the effective absorption of the marker into the cancerous lesion, precisely indicating its location. The product enhances convenience and utility by allowing real-time tracking and accurate confirmation of the extent of the lesion to be targeted for removal. The company emphasizes that Luminomark injection is an improved pharmaceutical agent contributing to the efficiency and effectiveness of the surgical procedure.
Jin-Sun Kim, Head of Research at Hanlim Pharmaceuticals, stated, "The challenges in conventional breast lesion removal surgery include lesions not being palpable, not being visible on the skin surface, and not visible to the naked eye during surgery," adding, "Luminomark injection addresses these issues by avoiding skin pigment deposition, maintaining low procedural difficulty, and providing a nearly pain-free treatment for patients."
Global Triumph in Technology Export Award for Chong Kun Dang, GI-Innovation, and OncoTherapeutics

Developed by Chong Kun Dang, CKD-510 is a low-molecular-weight compound targeting histone deacetylase 6 (HDAC6), incorporating the Non-Hydroxamate (NHA) platform technology. It stands as Chong Kun Dang's in-house R&D next-generation 바카라사이트 쇼미더벳 candidate. In November 2023, Chong Kun Dang secured a global technology export deal with Novartis, totaling $1.31 billion, including a contract fee of approximately $80.6 million and milestones of around $1.23 billion.
Director Chang-Sik Lee of Chong Kun Dang stated, "CKD-510 is the industry's first non-hydroxamate HDAC6 selective inhibitor. It stabilizes microtubules through HDAC6 inhibition, normalizing cellular functions." He added, "CKD-510 possesses a dual treatment mechanism that improves both electrical and structural transformations, exhibiting anti-fibrotic effects due to enhanced signaling, improved cell death, and inhibition of essential protein modifications in cells."
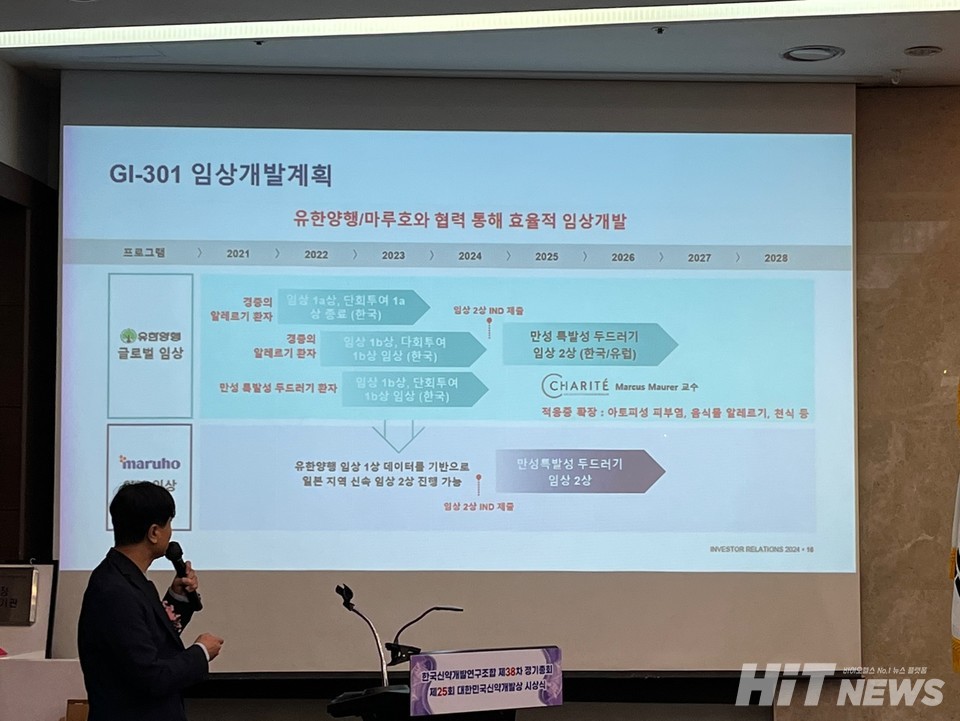
Developed by GI-Innovation, GI-301 is a dual-fusion protein new 바카라사이트 쇼미더벳 candidate designed using a platform that enhances 바카라사이트 쇼미더벳 safety by maintaining prolonged half-life function in the body while eliminating the antibody's inherent cell-killing function. In July 2020, the company signed a global technology transfer agreement, excluding Japan, with Yuhan Pharmaceuticals for preclinical stages, amounting to a total of $1.1 billion. In October 2023, GI-Innovation further signed a technology transfer agreement with the Japanese pharmaceutical company Maruho for development and commercialization within Japan, totaling $239 million, during the Phase 1 clinical trial stage.
Myung-Ho Jang, Chief Strategy Officer (CSO) at GI-Innovation, emphasized, "While the existing allergy treatment 'Xolair' is well-received in the market, it is unresponsive to patients with high levels of Immunoglobulin E (IgE)." He added, "GI-301 has demonstrated efficacy in reducing lung inflammation and fibrosis in allergy and asthma models. It is a treatment option that secures therapeutic efficacy and safety compared to competing drugs. Through value-driven R&D, we have achieved success in the technology export of GI-301."
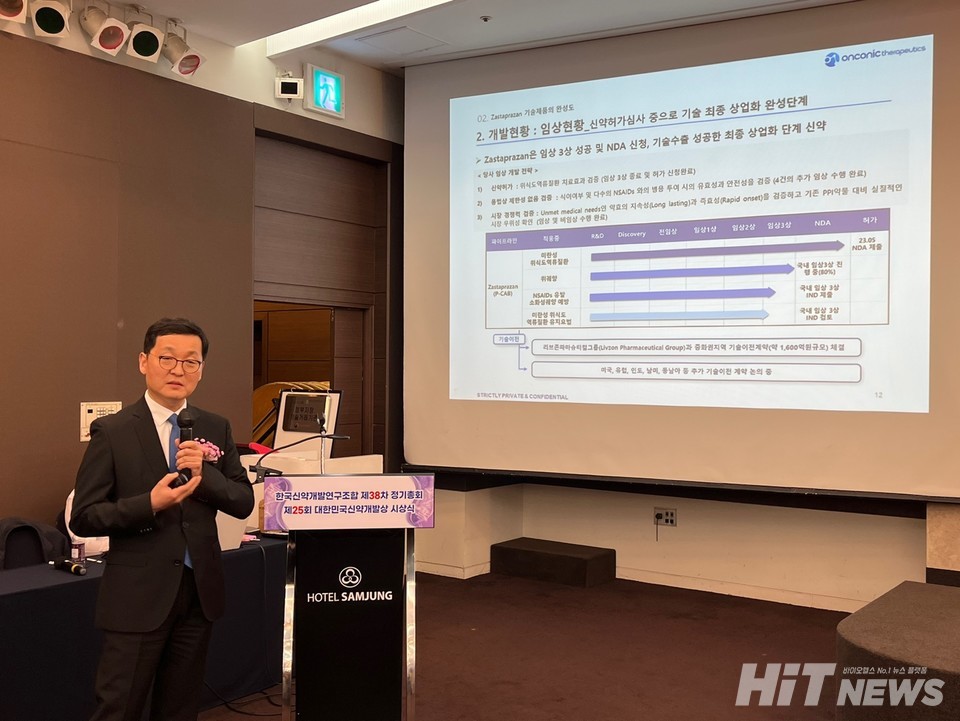
Developed by Onconic Therapeutics, Zastaprazan is a next-generation potassium-competitive acid blocker (P-CAB) candidate expected to replace traditional proton pump inhibitors (PPIs) in the market for digestive ulcer treatments, including gastroesophageal reflux disease. Zastaprazan, undergoing Phase 3 clinical trials for patients with erosive esophagitis, has demonstrated superiority in efficacy and safety, particularly in the treatment of mucosal lesions. The 바카라사이트 쇼미더벳 is anticipated to be a promising addition to the acid secretion inhibitors market.
In March 2023, Onconic Therapeutics signed a technology export agreement with the Livzon Pharmaceutical Group for the 바카라사이트 쇼미더벳 and commercialization of Zastaprazan, amounting to a total of $127.5 million. The company received a non-refundable upfront payment of $15 million and plans to continue advancing overseas business 바카라사이트 쇼미더벳 in the future.
John Kim, the CEO of Onconic Therapeutics, stated, "Zastaprazan is a novel drug candidate that meets the unmet medical needs in the digestive ulcer market with its rapid and long-lasting efficacy." He explained, "Through successful achievement of the primary efficacy endpoints in 204 patients during Phase 2 and 300 patients in Phase 3 clinical trials, we have confirmed its excellent efficacy and safety profile."
관련바카라사이트 쇼미더벳
- 신약개발 대상받은 렉라자와 알리글로, "글로벌로 영토 확장"
- 100주년 앞 둔 유한양행… 정기 주총서 회장·부회장 자리 신설한다
- 본업·자회사 성장 유한양행, 렉라자 앞세워 매출 '2조원' 도전
- 르포 | 면역글로불린 제제 '알리글로' 생산 GC녹십자 공장, 깐깐했다
- GC녹십자 '알리글로' 대한민국신약개발 부문 대상 수상
- 한림제약, 한국퀀텀컴퓨팅과 신약 공동 연구 계약 체결
- 노바티스는 왜, 계약금 1061억 주고 종근당 CKD-510을 탐냈나
- 종근당, 노바티스와 글로벌 기술수출 계약…13억500만달러 규모
- GI이노베이션 알레르기 신약, 대한민국신약개발상 기술수출상 수상
- 지아이이노베이션, 日 마루호에 알레르기 치료제 'GI-301' 기술이전
- 'P-CAB 신약개발' 온코닉테라퓨틱스, 기평 통과…"연내 코스닥 상장"
- 국산신약 37호와 IPO... 두 토끼 사냥 나선 온코닉테라퓨틱스
- 유한양행, 美 AACR서 면역항암제 2종 비임상 결과 포스터 발표
