Total of 13 Medications Newly Included or Coverage Expanded Until March This Year
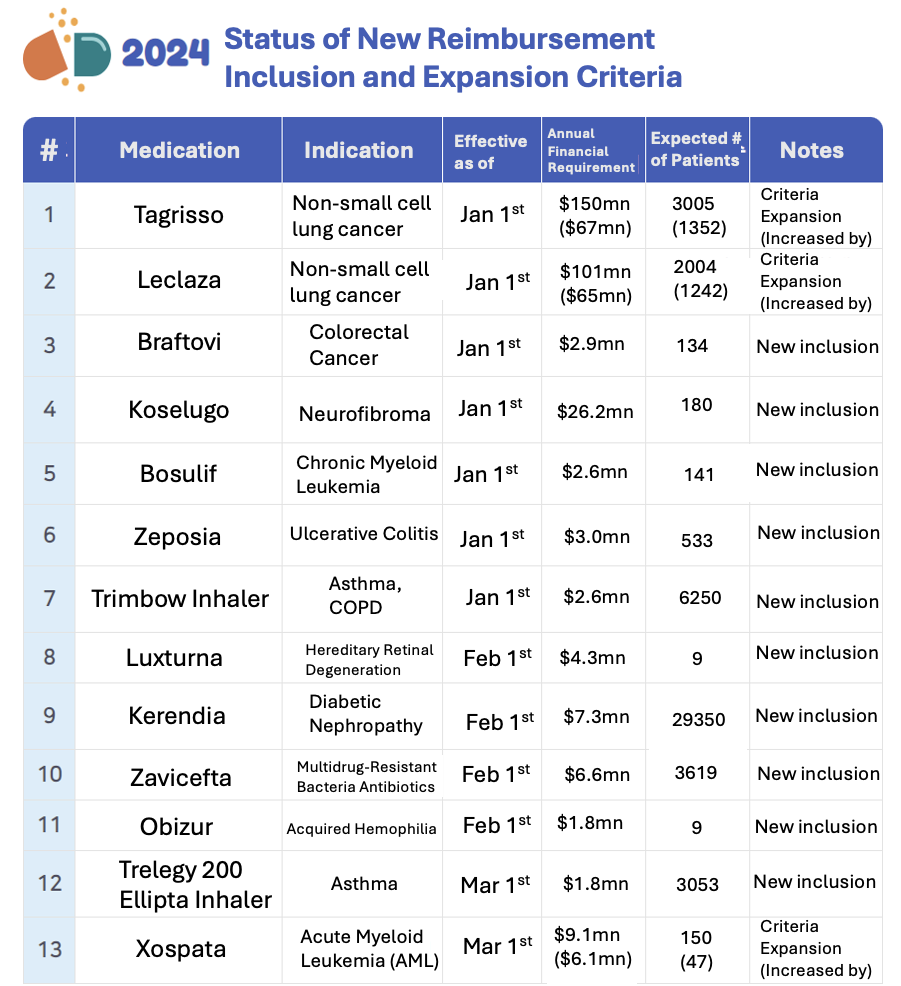
In March of this year, the Ministry of Health and Welfare disclosed the medications with the highest financial implications among those newly included or expanded in coverage.
A total of 13 medications, comprising 10 newly included and 3 with expanded coverage, were identified. Notably, Tagrisso and Leclaza emerged with the highest projected annual financial requirements. Tagrisso, by AstraZeneca Korea, is anticipated to incur $150 million annually, while Leclaza, developed by Yuhan Corp., follows closely with an estimated annual cost of $101 million. These figures represent considerable increases from before their coverage expansions. Tagrisso and Leclaza's expanded coverage encompasses their usage as first-line treatments for locally advanced or metastatic non-small cell lung cancer with specific genetic mutations. The coverage expansion also factors in their identification through genetic testing.
Additionally, Xospata garners attention for its substantial financial requirement following coverage expansion. Its expanded usage as a standalone therapy for relapsed/refractory acute myeloid leukemia (AML) irrespective of FLT3 mutations now qualifies for reimbursement, amounting to an estimated $9.1 million annually.
Among the newly included medications, Koselugo boasts the highest estimated financial requirement, totaling $27.6 million. Koselugo is earmarked for treating neurofibromatosis type 1 (NF1) associated with inoperable plexiform neurofibromas in patients aged 3 to 18 years old.
Conversely, Kerendia emerges as the most utilized medication, with an estimated patient count of 29,350. This medication is reimbursed for adult patients with chronic kidney disease and type 2 diabetes who exhibit specific criteria despite stable treatment with ACE inhibitors or angiotensin II receptor blockers for a minimum of 4 weeks.
Trimbow Inhaler and Zavicefta Patch, though with smaller patient populations, also highlight notable figures. The estimated number of eligible patients for reimbursement stands at 6,250 for Trimbow Inhaler and 3,619 for Zavicefta Patch, respectively. These insights into the financial implications and patient populations shed light on the evolving landscape of medication coverage and usage.
