Expanded Reimbursement for First-Line Lung Cancer Treatment
8-Month Prescription Revenue Matches Last Year’s Total
The EGFR mutation-targeted therapies for non-small cell lung cancer (NSCLC), 사설 바카라 (ingredient: Osimertinib) and Leclaza (ingredient: Lazertinib), have shown concurrent growth following the expansion of first-line reimbursement criteria.
According to pharmaceutical research firm UBIST, AstraZeneca's 사설 바카라 recorded .7 million in prescription revenue up to August this year. Prescription revenue steadily increased from .4 million in 2020 to .6 million in 2021 and .5 million in 2022. However, the introduction of South Korea's Leclaza into clinical practice led to a slight dip in 사설 바카라's revenue, down to .8 million in 2023.
Following the expansion of its indication for first-line lung cancer treatment in January, 사설 바카라 saw a significant surge in revenue. Cumulative revenue from January to August has already reached .7 million, nearly matching the total from last year. The drug’s price reduction has also encouraged its use as adjuvant therapy in early-stage EGFR-mutated NSCLC patients after surgery. With four months remaining in the year, 사설 바카라's total revenue is expected to surpass .7 million.
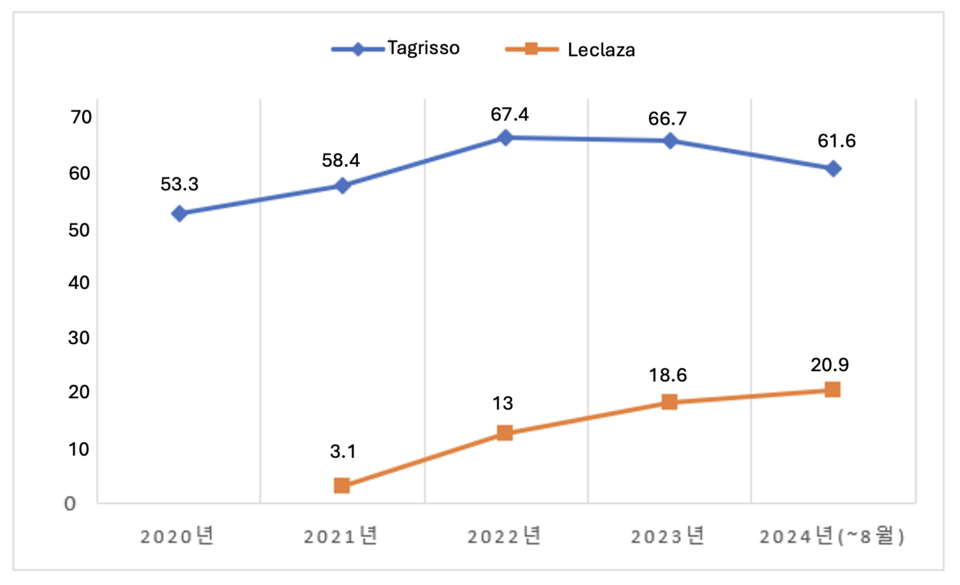
사설 바카라 has also experienced rapid growth, with prescription revenue climbing from .1 million in 2021 to million in 2022 and .7 million in 2023. From January to August this year, 사설 바카라 has already generated million, exceeding last year's total.
This growth is largely attributed to the expanded reimbursement for first-line lung cancer treatment, initiated in January. Furthermore, Leclaza has gained attention for its combination therapy with Rybrevant (ingredient: Amivantamab). A study presented at the World Conference on Lung Cancer (WCLC 2024) showed that patients with EGFR-mutated NSCLC (exon 19 deletion or L858R) achieved higher 3-year progression-free survival rates with the Rybrevant + Leclaza combination compared to 사설 바카라 monotherapy.
At 24 months, survival rates were 75% (95% CI: 71-79) for the Rybrevant + Leclaza group, compared to 70% (95% CI: 65-74) for the 사설 바카라 group. By 36 months, the gap widened further, with survival rates of 61% (95% CI: 56-67) for the combination therapy and 53% (95% CI: 47-59) for 사설 바카라.
On August 20, the FDA approved the Rybrevant + Leclaza combination therapy. Further data from the MARIPOSA-2 trial, comparing the combination of Rybrevant and chemotherapy against chemotherapy alone in EGFR-mutant advanced NSCLC patients who have progressed after 사설 바카라 treatment, will be presented at the European Society for Medical Oncology (ESMO 2024) conference on September 13.
