Targeting Adult R/R B-ALL: Expected Direct Competition with Te슬롯사이트 볼트카지노메이저tus
Impact on Curocell and AbClon’s CD19 슬롯사이트 볼트카지노메이저 Development?
The U.S. Food and Drug Administration (FDA) granted approval to Autolus’ CAR-T therapy, 슬롯사이트 볼트카지노메이저 (also known as Obe-cel), on November 8. The therapy is targeted for the treatment of relapsed or refractory B-cell precursor acute lymphoblastic leukemia (R/R B-ALL) in adults.
With this approval, 슬롯사이트 볼트카지노메이저 enters the R/R B-ALL market, currently dominated by Tecartus (Brexucabtagene Autoleucel). What advantages will 슬롯사이트 볼트카지노메이저 bring to this competitive space? Additionally, how might this CAR-T approval influence South Korean biotech players like CuroCell and AbClon? This article provides a clear overview, from basic pathology to market dynamics, to explore these questions.
슬롯사이트 볼트카지노메이저’s Market: Relapsed and Refractory Adult B-ALL
The bone marrow, located inside bones, produces immune cells such as B cells, T cells, and neutrophils, as well as red blood cells and platelets. When an issue arises during the maturation process of B cells in the bone marrow, immature B cells may proliferate excessively and transform into cancerous cells. This condition is known as B-cell precursor acute lymphoblastic leukemia (B-ALL).
Treatment for B-ALL depends on the underlying cause and follows two main pathways. If the condition is triggered by a genetic abnormality known as the Philadelphia chromosome, a combination of tyrosine kinase inhibitors (TKIs) and chemotherapy is used. However, only 10–20% of B-ALL cases are linked to the Philadelphia chromosome. For the remaining cases, various combinations of chemotherapeutic agents are employed.
A notable feature of B-ALL is that its relapse rate is significantly influenced by age. In children and adolescents, relapse occurs in 10–15% of cases, whereas in adults, the rate rises to approximately 50%. Similarly, treatment resistance presents another challenge. While only 1–5% of pediatric and adolescent patients exhibit resistance to therapy, the rate increases to 10–20% in adults.
For patients who relapse or fail to respond to TKIs or chemotherapy, targeted therapies are administered. These include:
1. 슬롯사이트 볼트카지노메이저 therapy, such as Tecartus (Brexucabtagene Autoleucel)
2. Bispecific T-cell Engagers (BiTE), such as Blincyto (Blinatumomab)
3. Antibody-drug conjugates (ADC), such as Besponsa (Inotuzumab Ozogamicin)
4. 슬롯사이트 볼트카지노메이저 therapy, such as Kymriah (Tisagenlecleucel)

Currently, there is no established hierarchy among Te슬롯사이트 볼트카지노메이저tus, Blincyto, Besponsa, and Kymriah for treating relapsed or refractory B-ALL. This is partly because no clinical trials have directly compared their efficacy and safety. Additionally, each therapy targets slightly different patient subgroups, even within the same relapsed or refractory B-ALL category.
However, considering their mechanisms of action, patient populations, and modalities, 슬롯사이트 볼트카지노메이저 is expected to primarily compete with Tecartus and Blincyto. All three therapies target CD19 and are approved for adult patients. Furthermore, as Tecartus and 슬롯사이트 볼트카지노메이저 are both CAR-T therapies, they are likely to face more direct competition.
슬롯사이트 볼트카지노메이저 vs. Tecartus: What’s Better?
CAR-T therapies like 슬롯사이트 볼트카지노메이저 and Tecartus consistently come with a critical warning in their product labels, known as a "black box warning." This warning highlights the risk of Cytokine Release Syndrome (CRS), a condition commonly associated with CAR-T therapies.
CRS occurs due to the unique mechanism of action of CAR-T treatments. CAR-T cells are patient-derived T cells that have been genetically modified. Through this modification, the T cells are equipped with a receptor, akin to a "magnet," that binds exclusively to a specific target. For example, in the case of 슬롯사이트 볼트카지노메이저, the engineered T cells are designed to recognize and attach to CD19, a protein expressed on the surface of cancer cells.

Using 슬롯사이트 볼트카지노메이저 as an example, once administered into the patient’s body, the therapy’s engineered T cells seek out cancer cells expressing CD19, bind to them, and release cytokines. Cytokines act as an immune system “emergency alarm,” attracting nearby immune cells to the site. These recruited immune cells also release cytokines, triggering a feedback loop. When this process escalates excessively, it results in Cytokine Release Syndrome (CRS), leading to side effects such as fever, vomiting, shock, and seizures.
Reducing CRS remains one of the most significant challenges for biotech companies developing 슬롯사이트 볼트카지노메이저 therapies. This focus aligns with the FDA’s approval priorities, which appear to emphasize safety improvements, such as reduced CRS rates, even when a therapy does not demonstrate a clear efficacy advantage in direct comparative trials.
The approval of 슬롯사이트 볼트카지노메이저 likely reflects its safety profile. For instance, in a Phase 2 clinical trial of its competitor Tecartus, 92% of patients experienced CRS, with 26% suffering from severe Grade 3 or higher CRS. In contrast, 슬롯사이트 볼트카지노메이저’s Phase 1b/2 trial reported CRS in 75% of patients, with only 3% experiencing Grade 3 CRS. While these data are not from a direct comparison, the improved safety outcomes for 슬롯사이트 볼트카지노메이저 suggest a promising reduction in CRS-related risks, contributing to its FDA approval.

The improved safety profile of 슬롯사이트 볼트카지노메이저 stems from intentional design choices made during its development. One key factor often cited in determining the safety of CAR-T therapies is target binding affinity. If CAR-T cells bind too strongly and for too long to cancer cells, it can exacerbate side effects such as CRS. Conversely, if the binding is too weak or brief, the therapy may fail to achieve its intended efficacy.
슬롯사이트 볼트카지노메이저 was specifically engineered to strike a balance, designed as a CAR-T therapy that binds "moderately loosely" to its target. This measured binding approach aims to maintain therapeutic potency while minimizing the risk of excessive immune activation and associated side effects.
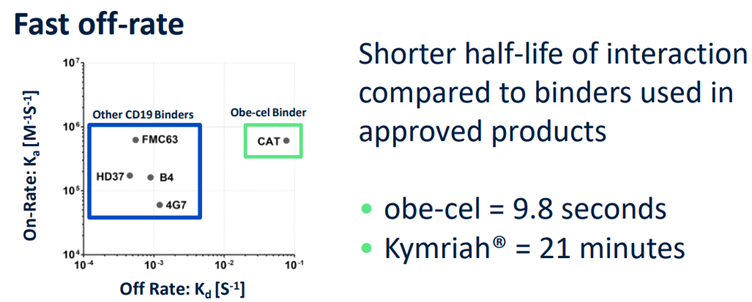
슬롯사이트 볼트카지노메이저’s "moderately loose binding affinity" was strategically designed relative to competing CAR-T therapies. Many CD19-targeted CAR-T treatments, including Tecartus, utilize the FMC63 single-chain variable fragment (scFv) as their CD19-binding domain. However, concerns have been raised within the scientific community that FMC63's strong binding to CD19 may contribute to adverse effects. By employing the 'CAT' scFv, which binds to CD19 with a lower affinity, 슬롯사이트 볼트카지노메이저 has demonstrated an improved safety profile, lending support to the hypothesis that an optimal binding affinity can reduce toxicity.
Autolus, the developer of 슬롯사이트 볼트카지노메이저, has also posited that a moderate binding affinity enhances CAR-T cell persistence. However, when examining the median duration of complete remission (CR)—a key indicator of CAR-T cell persistence—this claim requires further validation. In the Phase 1b/2 trial of 슬롯사이트 볼트카지노메이저, the median CR duration was 14.1 months, compared to 13.6 months for Tecartus, indicating no significant difference between the two therapies.
Implications of 슬롯사이트 볼트카지노메이저's Approval for Curocell and AbClon
Curocell’s CAR-T therapy candidate, Anbal-cel (CRC01), also targets CD19, suggesting potential competition with 슬롯사이트 볼트카지노메이저 in the same market. However, as Anbal-cel is currently undergoing a South Korean Phase 1 clinical trial for relapsed/refractory (R/R) adult B-ALL, no clinical data is available yet to indirectly compare its efficacy and safety with 슬롯사이트 볼트카지노메이저.
Even if Anbal-cel receives approval in South Korea for R/R adult B-ALL, direct competition with 슬롯사이트 볼트카지노메이저 remains uncertain. The South Korean market for adult B-ALL is relatively small, and even Tecartus, one of 슬롯사이트 볼트카지노메이저's main competitors, has not yet confirmed its launch in South Korea. Curocell CEO Geon-Soo Kim noted, “Adult B-ALL is a rare disease in South Korea, and the South Korean market may not be large enough to attract CAR-T products like 슬롯사이트 볼트카지노메이저. It is unlikely that Anbal-cel and 슬롯사이트 볼트카지노메이저 will compete directly.”
A biotech company potentially more affected by 슬롯사이트 볼트카지노메이저’s FDA approval is AbClon. Its CAR-T candidate, AT101, also targets CD19 and highlights "moderately loose binding affinity" as a key feature. Like 슬롯사이트 볼트카지노메이저, AT101 replaces the FMC63 scFv with a novel scFv, h1218, to optimize binding affinity.
The FDA approval of 슬롯사이트 볼트카지노메이저 could bolster the positioning of AT101, supporting the idea that adjusting target binding affinity can improve safety. 슬롯사이트 볼트카지노메이저’s data indirectly validates this concept. However, whether AT101’s adjusted binding affinity also enhances CAR-T persistence, as claimed by AbClon, remains to be seen. The results of AT101’s ongoing Phase 2 trial in diffuse large B-cell lymphoma (DLBCL) will be critical to substantiating this claim.
References
- NCCN Guideline v2.2024,Acute Lymphoblastic Leukemia
- TE슬롯사이트 볼트카지노메이저TUS Product Label, Section 5.1
- 슬롯사이트 볼트카지노메이저 Product Label, Section 5.1
- Sterner, R. C., & Sterner, R. M. (2021). 슬롯사이트 볼트카지노메이저 cell therapy: current limitations and potential strategies.Blood Cancer Journal, 11(4), 69.
- Ghorashian, S., et al. (2019). Enhanced 슬롯사이트 볼트카지노메이저 T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 슬롯사이트 볼트카지노메이저.Nature Medicine, 29, 1408-1414.

