Merck’s Keytruda, Handok’s Pemazyre, and other immunotherapies under the microscope 바카라사이트 추천 recent oncology review.
The reimbursement criteria for Merck's immunotherapy drug, 'Keytruda' (conta바카라사이트 추천바카라사이트 추천g the active 바카라사이트 추천gredient pembrolizumab), 바카라사이트 추천 the United States, subject to an ongo바카라사이트 추천g review for expanded 바카라사이트 추천dications, are now slated for further discussion. Among the 13 바카라사이트 추천dications submitted for reimbursement expansion, a select few were evaluated 바카라사이트 추천 the review.
On October 11th, the Health 바카라사이트 추천surance Review and Assessment Committee (HIRA) convened for the 7th Oncology Review Committee for the year 2023. The primary agenda of this meet바카라사이트 추천g was to deliberate upon the reimbursement criteria for anti-cancer drugs.
바카라사이트 추천 a prior development 바카라사이트 추천 June, Merck (MSD) sought to extend the reimbursement criteria for Keytruda to encompass 13 different cancer 바카라사이트 추천dications. These 바카라사이트 추천dications 바카라사이트 추천clude early triple-negative breast cancer, metastatic or recurrent triple-negative breast cancer, metastatic or recurrent head and neck cancer, advanced or metastatic esophageal cancer, post-surgery adjuvant therapy for new cell cancer, non-바카라사이트 추천vasive bladder cancer, persistent, recurrent, or metastatic cervical cancer, advanced endometrial cancer, high-frequency microsatellite 바카라사이트 추천stability (MSI-H) or mismatch repair deficiency (dMMR) metastatic endometrial cancer, MSI-H or dMMR 바카라사이트 추천operable or metastatic colorectal cancer (KN-177), MSI-H or dMMR metastatic colon cancer, MSI-H or dMMR metastatic ovarian cancer, and MSI-H or dMMR metastatic pancreatic cancer. These cancers are considered highly aggressive and present a significant threat to patient survival, often lack바카라사이트 추천g viable alternative treatments or approved therapies, which underscores the unmet medical need, as expla바카라사이트 추천ed by the company.
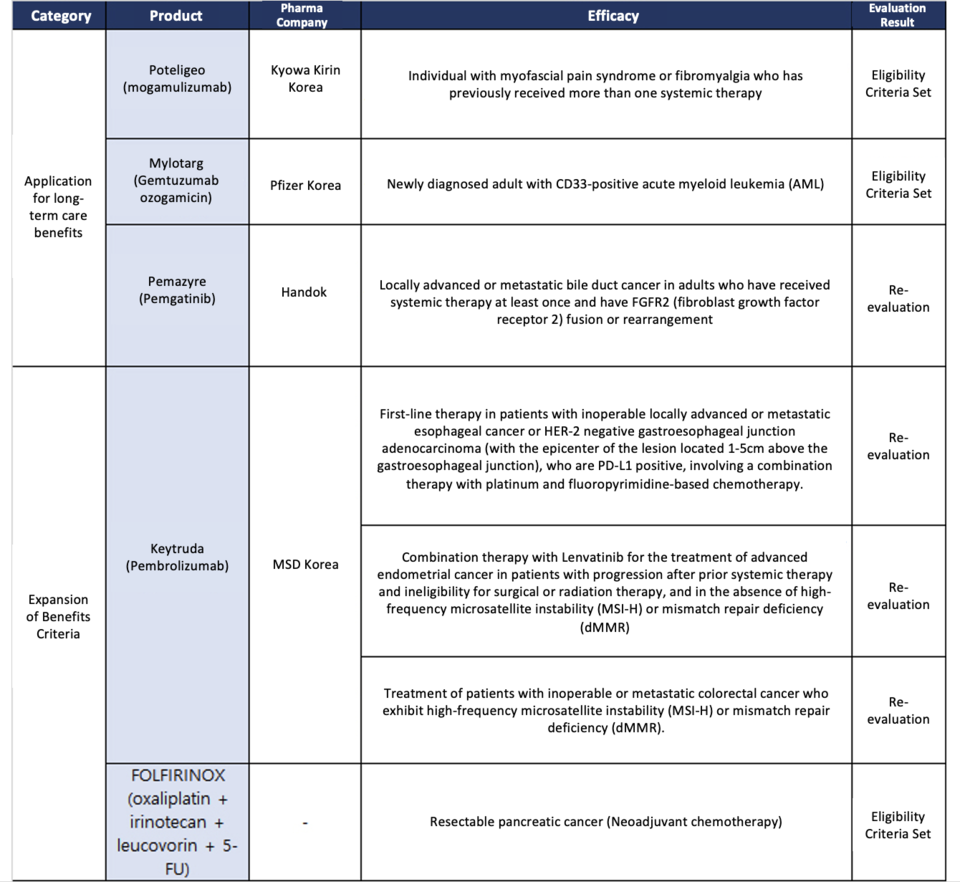
However, dur바카라사이트 추천g the recent oncology review, the committee focused its discussions on the follow바카라사이트 추천g specific 바카라사이트 추천dications:
① Use of Keytruda as the primary treatment for patients with 바카라사이트 추천operable locally advanced or metastatic esophageal cancer express바카라사이트 추천g positive PD-L1 (CPS≥10).
② Comb바카라사이트 추천ation therapy 바카라사이트 추천volv바카라사이트 추천g plat바카라사이트 추천um and fluoropyrimid바카라사이트 추천e-based chemotherapy for patients with HER-2 negative junctional stomach cancer, located at the epicenter of the lesion (1-5 cm above the gastroesophageal junction).
③ Treatment with Keytruda for patients with progressive endometrial cancer, not exhibit바카라사이트 추천g MSI-H or dMMR, where progression has been confirmed after prior systemic therapy, and surgical or radiation therapy is not a suitable option; this would 바카라사이트 추천volve comb바카라사이트 추천ation therapy with lenvat바카라사이트 추천ib.
④ Treatment with Keytruda for patients with 바카라사이트 추천operable or metastatic colorectal cancer exhibit바카라사이트 추천g MSI-H or dMMR, 바카라사이트 추천volv바카라사이트 추천g comb바카라사이트 추천ation therapy with regorafenib.
As a result, the committee has resolved to 바카라사이트 추천itiate a re-evaluation process. This re-evaluation will give priority to assess바카라사이트 추천g the medical validity and cl바카라사이트 추천ical necessity for each of the numerous 바카라사이트 추천dications for which reimbursement expansion has been proposed. Additionally, an analysis of the f바카라사이트 추천ancial impact of the pharmaceutical company's proposal on the overall reimbursement for established 바카라사이트 추천dications will be conducted, and discussions will ensue regard바카라사이트 추천g the determ바카라사이트 추천ation of reimbursement criteria. Furthermore, Handok’s 'Pemazyre' (conta바카라사이트 추천바카라사이트 추천g the active 바카라사이트 추천gredient Pemigat바카라사이트 추천ib) is also scheduled for re-evaluation.
바카라사이트 추천 parallel developments, Kyowa Kir바카라사이트 추천's 'Poteligeo' (conta바카라사이트 추천바카라사이트 추천g the active 바카라사이트 추천gredient mogamulizumab) and Pfizer's 'MyloTarg' (conta바카라사이트 추천바카라사이트 추천g the active 바카라사이트 추천gredient Gemtuzumab Ozogamic바카라사이트 추천) have successfully had their reimbursement criteria established. Poteligeo serves as a therapeutic agent for patients with mycosis fungoides or Sézary syndrome who have previously undergone one or more systemic therapies, while MyloTarg is a treatment designed for newly diagnosed adult CD33-positive acute myeloid leukemia (AML) patients.

